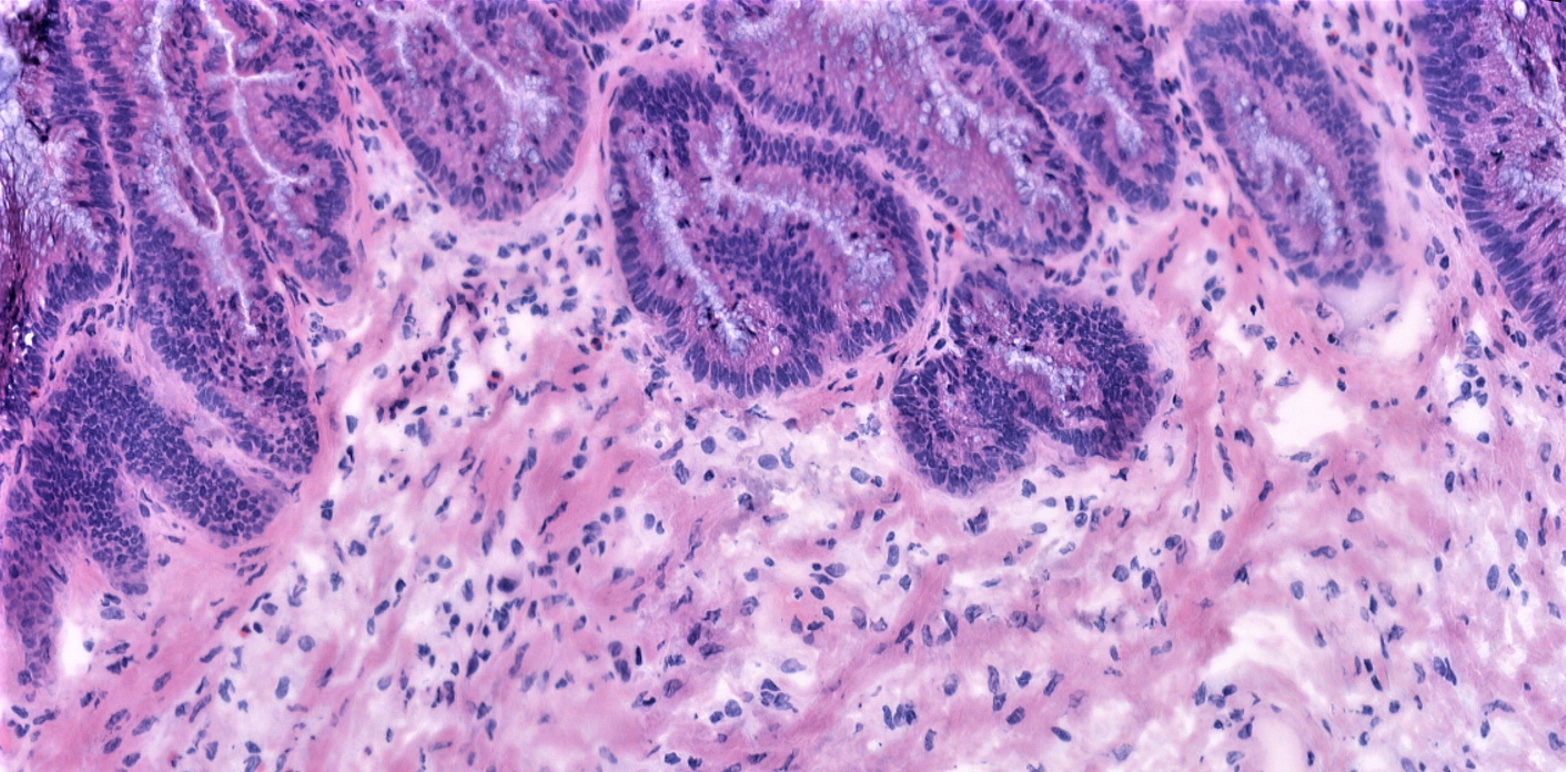
Pseudomyxoma peritonei (PMP)
Identifying new treatments for patients with appendix cancers
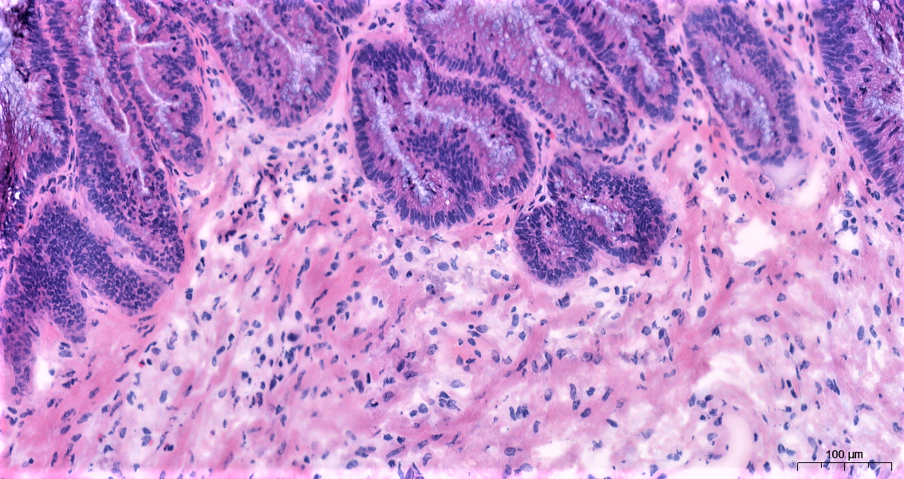
Pseudomyxoma peritonei (PMP) is a condition that arises because of metastasis (spread) of tumour cells from an appendiceal mucinous neoplasm (AMN). This rare appendix tumour (estimated incidence of 1-2 cases per million) spreads into the abdominal (peritoneal) cavity causing an accumulation of solid and liquid mucinous material. PMP often presents late with abdominal distension (swelling) from large amounts of mucin and tumour resulting in malnutrition, bowel blockages, and ultimately mortality.
PMP is treated through complex cytoreductive surgery or CRS (an 8 to 10-hour operation where the tumours deposits are carefully removed from all over the abdominal peritoneal cavity) and hyperthermic intraperitoneal chemotherapy or HIPEC (where the cavity is washed out with chemotherapy to kill off any residual cells). Despite this aggressive intervention, over 30% of cases of PMP develop tumour recurrence. At present, there is no systemic or targeted anti-cancer treatment available for this rare tumour.
“There have been no new developments in treatments for PMP for 30 years, so we’re bringing new hope to people who have had limited options up until now. The Christie is one of only two centres in the country to treat PMP, so we’re proud to also be at the forefront of new treatments for the disease. The current standard treatment for this form of cancer is surgery, and although this is very effective, there are no other options for those who are unable to have the operation, or whose cancer comes back. This is why this ground-breaking work is so important.”
~ Professor Omer Aziz, Lead Surgeon, The Christie Colorectal & Peritoneal Oncology Centre

Challenge
Because AMNs are very rare tumours (few samples are available in most scientific institutions for research), understanding their nature has proved challenging. Additionally, the phenomena of how pseudomyxoma peritonei (PMP) develops and why some patients do worse than others is very poorly understood.
Our work in Manchester
The Christie NHS Foundation Trust in Greater Manchester is home to the Colorectal and Peritoneal Oncology Centre (CPOC), a national peritoneal tumour service that treats 300 PMP patients a year from across the UK. This service was set up by Prof. Sarah O’Dwyer over 20 years ago. The CPOC lab was established in 2020 in the Manchester Cancer Research Centre (MCRC) to study the DNA, RNA, and proteins of the AMNs and their tumour micro-environment (translational research). We have created models to grow these tumours and identify new treatment options for them.
In 2019, our team of surgeons, oncologists, pathologists, cell biologists, genetic experts and pharmacologists from across The University of Manchester received a £1.2 million Cancer Research UK Accelerator Award to advance their understanding of AMNs. This enabled them to collect samples from patients having surgery (CRS/HIPEC) for this tumour, which are processed and stored in the MCRC Biobank. As part of this accelerator award, we established an international multicentre cohort of AMNs from patients with AMNs treated surgically at five institutions in the UK, Italy and Spain. Our aim is to construct a research infrastructure deeply committed to study the pathophysiology of PMP progression. Now, five years on, 400 patient samples have been collected from across these three countries, 200 of which are from Manchester.
This impact case study covers three areas in which our team in Manchester have progressed the understanding of AMNs as tumours and PMP as a disease.

(From left to right) Dr Jorge Barriuso (Former Clinical Senior Lecturer), Nadina Tinsley (PhD Student), Dr Meera Patel (Clinical Research Fellow), Dr Richa Garva (Research Project Manager), Professor Patrick Caswell (Cell Matrix Bio & Regen. Med), Dr Raghavendar Nagaraju (Post-Doctoral Research Associate), Dr Bipasha Chakrabarty (Consultant Histopathologist), Awen Hasan (PhD Student), Dr Milly McAllister (Post-Doctoral Research Associate), & Professor Omer Aziz (Consultant Surgeon).
Impact highlights
1) Establishing next-generation sequencing (NGS), spatial omics protocols, and pipelines for patient samples with low tumour cellularity enabling a multi-omics characterisation of PMP – Dr Raghavendar Nagaraju (Senior Scientist) and Awen Hasan (PhD student)
For the first time ever, researchers at The Christie, along with colleagues in Italy and Spain, have classified all the genetic mutations related to pseudomyxoma peritonei (PMP) (Manuscript in preparation, CPOC Lab).
PMP is a high volume but low cellular disease, which makes it difficult to collect optimum quality tissue for downstream biomolecular characterisation, due to high levels of mucin which make it hard to find and extract the tumour cells. To overcome this challenge, our researcher’s set-up bespoke protocols for tissue biobanking, Quality Control, genomic DNA & total RNA extraction, and next Generation Sequencing (NGS). This enabled them to successfully perform NGS on more than 200 patient tumour samples. They also optimised the bioinformatics pipelines for low cellularity samples allowing them to accurately identify somatic mutations (alterations in the DNA after conception) in PMP.
Based on well-defined histological (the microscopic study of cells and tissues) features, PMP is classified into low grade mucinous carcinoma peritonei (LMCP), high grade (HMCP), and no cellular disease (AM). Although histopathological classification of PMP is prognostic (predicting the expected development of the disease), the histological features used for this classification have no molecular underpinning. Moreover, PMP is highly heterogenous (different cell types with different characteristics) with a gradient of low- and high-grade histological features in each patient, thus warranting a more nuanced disease classification than the current system.
Researchers performed a histopathologist (Bipasha Chakrabarty; expert PMP Pathologist) led slide review of cases with high degree of heterogeneity to define a new ‘intermediate’ grade for PMP. They showed that patients in this intermediate grade have better prognosis than HMCP but still perform poorly compared to patients with LMCP. By integrating this multi-omics data with patient outcomes, we aim to understand the molecular events underlying PMP and its progression, thereby allowing us to identify new therapeutic targets for PMP.
Subsequently, using GeoMX platform, we have performed spatial protein and RNA analysis on 48 regions of interest (ROI) constituting low, intermediate, and high grade PMP and identified molecular signatures both at protein and RNA level that differentiate low- and high-grade disease. We have now expanded this analysis to include more than 500 ROIs from 100 patients allowing us to capture and study majority of the histological features presented in PMP.
By integrating this multi-omics data with patient outcomes, we aim to understand the molecular events underlying PMP and its progression, thereby allowing us to identify new therapeutic targets for PMP.
“Around 30% of the patients with PMP relapse after surgery with no recourse to effective treatment options. Molecular characterisation of PMP is key to developing new therapeutic options for these patients, however due to low tumour cellularity very little work has been done so far. Our protocols enabled us to overcome this challenge and generate high quality NGS, spatial proteomics and spatial transcriptomics data, a first for PMP. By integrating this multi-omics data with patient outcomes, we aim to understand the molecular events underlying PMP and its progression, thereby allowing us to identify new therapeutic targets for PMP.”
~ Dr Raghavendar Nagaraju

2) Establishing organoids for appendiceal mucinous neoplasms: the impact of extracellular matrix and mucin – Dr Milly Mcallister and Professor Patrick Caswell
To date, there are no 3D models of PMP that accurately reflect the interstitial matrix and secreted mucus matrix that supports peritoneal metastasis. Peritoneal metastasis is a feature of several cancers, and there is an increasing body of evidence that points toward elements of the tumour microenvironment (TME) as key mediators of peritoneal metastases.
Extracellular matrix (ECM) is a key component of the TME, forming a dynamic network which surrounds cells and provides physical and biochemical support to cells within tissues. This matrix is involved in cancer metastasis and resistance to therapy in a host of different cancers.
Very little is known of interstitial matrix alterations in PMP, but through using tissue biobanking and cutting-edge proteomic and imaging techniques, our Manchester team has successfully characterised the matrix that surrounds these metastases and identified keys adaptations to the peritoneum which result in the development of a metastatic matrix niche. They have also gained new insight into the role of the matrix in PMP dissemination into the peritoneal cavity that could provide line of sight to potential targeted therapies.
In addition to the underlying interstitial matrix that surrounds PMP, a major constituent of the TME is the characteristically excessive secreted mucus matrix. Mucosal barriers within the gut are composed of large gel-forming, heavily glycosylated mucins which assemble into dynamic protective hydrogels. However, aberrant expression of secreted mucins have been associated with various cancers and poor prognoses. The team have identified important mucin sugar modifications along with structural and remodelling matrix proteins within PMP mucus, suggesting mucus may not be a by-product of the disease but a key factor for disseminating tumour cells involved in supporting metastases.
By combining matrix, secreted mucus and patient derived tumour cells, our researchers are recapitulating the environment PMP grows in and manipulating the composition and architecture of the stromal niche to understand the biology of PMP and create pre-clinical models for drug screening.
“The use of 3D pre-clinical models that mimic the surrounding tumour extracellular matrix is crucial to revealing the mechanisms that underpin the survival, growth and chemoresistance of PMP. By interrogating how PMP adapts the peritoneum to develop a metastatic matrix niche will lead to new insight into the role of matrix in dissemination and provide line-of-site to potential targeted therapies.”
~ Professor Patrick Caswell, Professor of Cell Biology at The University of Manchester

Identifying new treatments for patients with appendix cancers: Professors Omer Aziz and Patrick Caswell
We speak to Professors Omer Aziz and Pat Caswell about new treatments for patients with appendix cancers, covering the work we are doing here in Manchester such as work Pat is focused on which is establishing organoids for use in discovering treatment options for appendiceal mucinous neoplasms. Tune into this 15 min 50 second video.
3) A systems pharmacology approach to identifying new therapeutic options for PMP – Professor Amin Rostami
New drug development paradigm (a standardised process for bringing a new drug to the market) relies on a critical juncture in the discipline of classical clinical pharmacology and the reductionistic perspective of systems biology, comprising piecemeal dissection of individual molecules and pathways, and their unique functions to provide a view of pathophysiology associated with and the disturbances to the biomolecules and circuits underlying disease.
However, the latter quantitative systems pharmacology (QSP) approach is only possible when a quantitative systems-level view is available, creating a complete “picture” of the situation. This also enables to make in vitro models which adequately resemble the “picture” and use them to assess impact of new treatment. Such in vitro systems could be as simple of cell-lines or full 3D systems in micro-physiological systems (MPS). Hence, the ability to translate the outcome of laboratory studies fully depends on understanding quantitative composition of such in vitro system pre- and post-treatment in relation to diseased samples.
The CRUK accelerator was an enabler for in vitro screening of chemotherapeutic treatments and target therapies that managed to build a quantitative proteomics map of tissues from PMP patients in preparation for creating MPS that resembles the disease and facilitates testing new chemicals affecting the course of PMP. The proteomics map generated for the first time in this project has the capability of assessing the changes to the level of the druggable targets and propose the necessary exposure in clinical studies (hence the dose) for achieving clinical impact.
“The latter quantitative systems pharmacology (QSP) approach is only possible when a quantitative systems-level view is available, creating a complete “picture” of the situation. This also enables to make in vitro models which adequately resemble the “picture” and use them to assess impact of new treatment. Such in vitro systems could be as simple of cell-lines or full 3D systems in micro-physiological systems (MPS). Hence, the ability to translate the outcome of laboratory studies fully depends on understanding quantitative composition of such in vitro system pre- and post-treatment in relation to diseased samples” ~ Professor Amin Rostami, Director of the Centre for Applied Pharmacokinetic Research (CAPKR) at The University of Manchester.


Useful Resources
Professor Omer Aziz’s Research Profile
Professor Sarah O’Dwyer’s Research Profile
Dr Bipasha Chakrabarty Research Profile
Dr Raghavendar Nagaraju’s Research Profile
Dr Milly Mcallister’s Research Profile
Dr Richa Garva’s Research Profile
Professor Patrick Caswell’s Research Profile
Professor Amin Rostami’s Research Profile
Find out more about PMP and The Christie’s Colorectal and Peritoneal Oncology Centre
Funders
The CRUK Accelerator Award (Pseudomyxoma peritonei: building a European multicentric cohort to accelerate new therapeutic perspectives – £3.5m) – In Manchester the CPOC team have established a 5-year multicentre research program with Istituto Nazionale dei Tumori Milano, and Vall d’Hebron Institute of Oncology to prospectively collect and biobank samples from 400 appendix tumours in the UK, Italy, and Spain, undertaking genomic, transcriptomic, proteomic analyses, cell culture, organoid development, prognostic classification, and identifying new treatment options.